Repulsive electron-electron interaction and nuclear charge screening: Ground state of two-electron atoms: Ndinya, Boniface, Akeyo, Joseph: 9783846540688: Amazon.com: Books
![PDF] Electron screening and excitonic condensation in double-layer graphene systems | Semantic Scholar PDF] Electron screening and excitonic condensation in double-layer graphene systems | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/12258383aed45faa8f3fb8f9277a44ffb3624dd7/1-Figure1-1.png)
PDF] Electron screening and excitonic condensation in double-layer graphene systems | Semantic Scholar
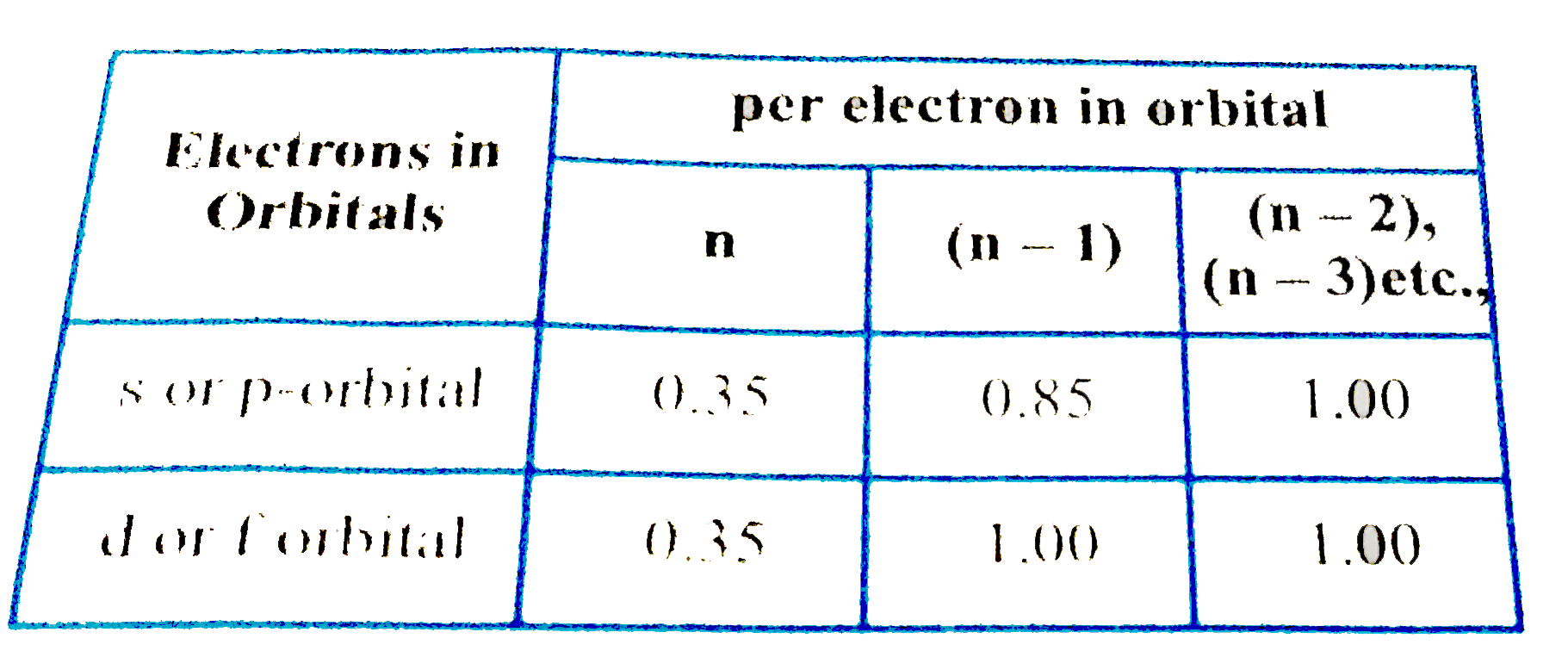
According to I.C slater effective nuclear charge, Z^(**), due to screening, is not exactly equal to the actual nuclear charge Z of the nucleus of the atom. Z^(**) depends on the type

Color online) (a) Valence-electron screening cloud around the excited... | Download Scientific Diagram

PDF) Electron screening effect in the reactions 3He(d, p) 4He and d( 3He, p) 4He Supported in part by INFN, BMBF (06BO812), DFG (436UNG113-146) and OTKA (T025465 | Matthias Junker -

Color online) (a) Valence-electron screening cloud around the excited... | Download Scientific Diagram

SOLVED: Question 37 (1 point) Screening of the nuclear charge by core electrons in atoms is: less efficient than by valence electrons more efficient than by valence electrons essentially identical to that
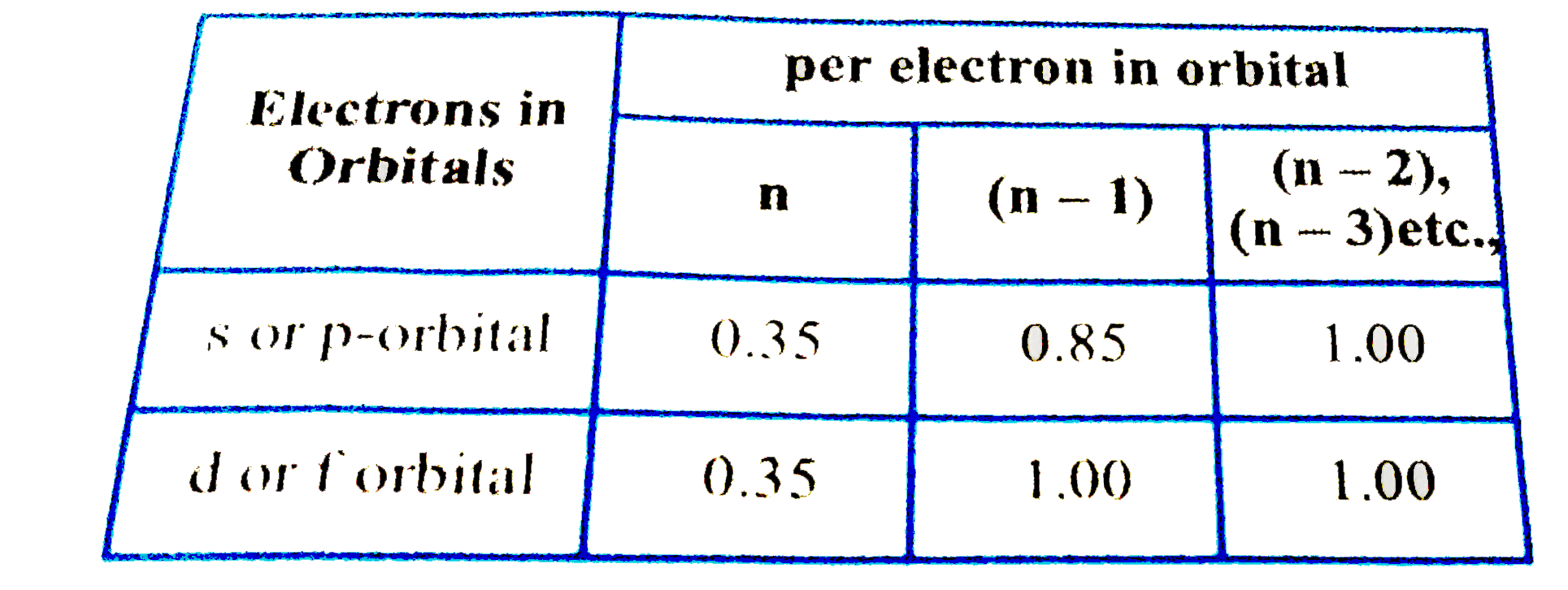
According to I.C slater effective nuclear charge, Z^(**), due to screening, is not exactly equal to the actual nuclear charge Z of the nucleus of the atom. Z^(**) depends on the type
Welcome to Chem Zipper.com......: Effective Nuclear charge (Z* or Zeff): Slater's rule: Screening effect or Shielding effect